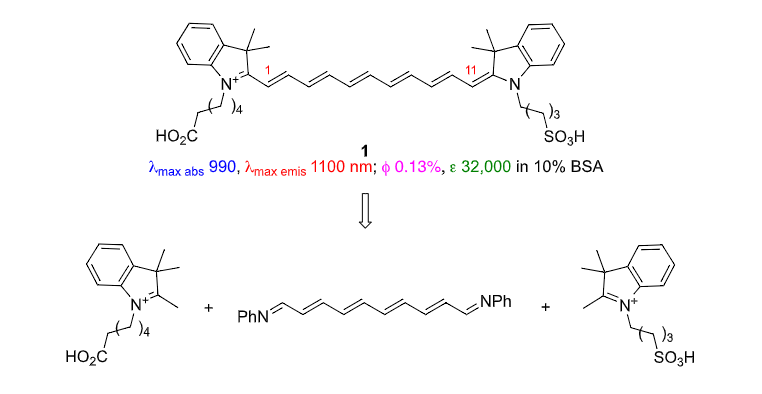
Cy11
Cy11 Homolog Of Indocyananine Green (ICG)
There are some water-soluble nearIR dyes, but very few nearIRII homologs, though
several groups are trying to make them. Sometimes the way forward is right under our
noses. Takashi Jin at the Center for Biosystems Dynamics Research, RIKEN, Suita,
Osaka, Japan instructed his group to make a Cy11 dye in almost exactly the same way
as ICG is synthesized. Routes like that shown in the Scheme below, but to ICG, are so
widely known that it is amazing that no one else had successfully extended them to the
Cy11 system 1, but Jin’s Bioconjugate Chem. (2021, acs.bioconjchem.1c00253) seems
to be the first report. Predictably, 1 has a poor quantum yield in water but, usefully, this
parameter increases to 0.13% in the presence of albumin. Under those conditions 1
has an l max abs of 990 nm and maximal fluorescence emission of 1010 nm; both about
200 nm red-shifted relative to ICG, which is a significant improvement with respect to
imaging in tissue. Indeed, in vivo experiments showed 1 injected into mice can be
imaged in cerebral capillaries, whereas ICG did not show, and conjugation to a mAb
targeting epidermal growth factor receptor (EGFR) then injection of this into mice
bearing HER2 + tumors imaged that lesion type. Overall, this is a breakthrough in the
field.
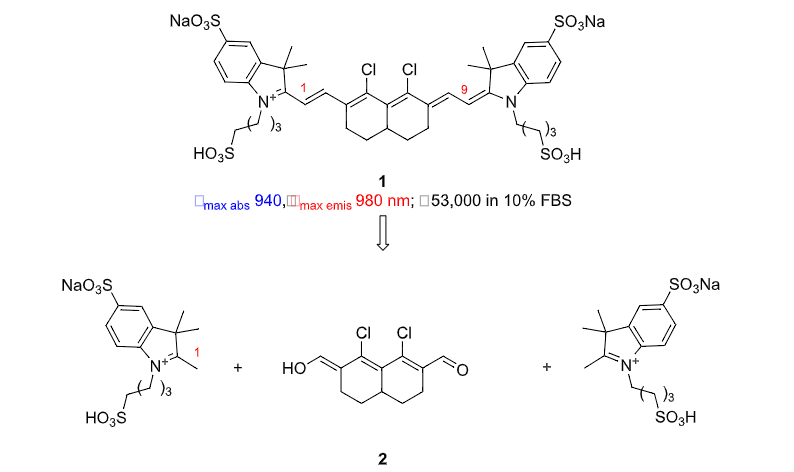
Cy9
Cy9 has emerged in the patent literature (US 2021/0179597 A1). The Scheme below
shows a synthetic route used to prepare the illustrative system 1 and an extensive
collection of related dyes; the origin of this innovation is the realization that the starting
material 2 is available via a Vilsmeyer-Haak reaction of the corresponding diketone.
Apparently, most of the dyes prepared are water-soluble, and a little fetal bovine serum
(FBS) probably helps (because it contains albumin). Electronic spectra of these dyes
are not much blue-shifted relative to the Cy11 system discussed above, but different
enough to provide potentially useful complementarity. Locked Cy9 systems including 2
probably have other useful properties too.
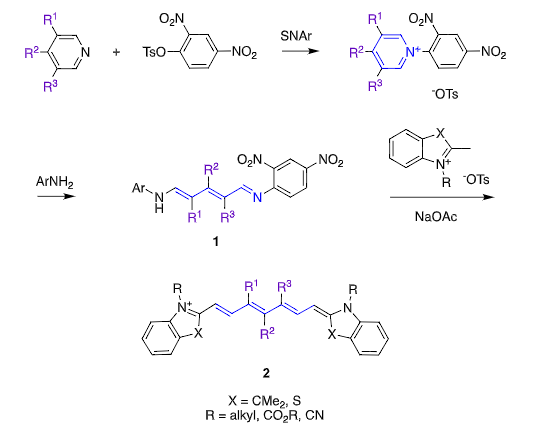
Cy7
New Cy7 Syntheses
A 2013 world patent (WO 2013/114115 Al) then J. Am. Chem. Soc. (2019,
jacs.9b02537) reports an unusual method to obtain cyanine-7 dyes. This approach
features the formation of pyridinium salts then ring opening with a nucleophile to give
electrophiles 1 in the special case wherein the nucleophile is a latent enamine from
heterocyclic salts. 3,4,5-Substitution of the pyridine is tolerable in this process, hence
correspondingly substituted cyanine-7 dyes 2 can be prepared, in some cases in a
multigram scale (Scheme).
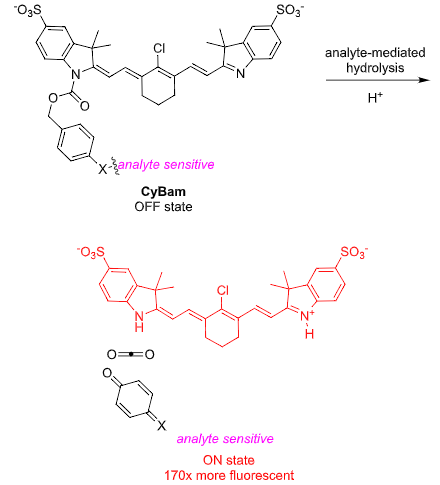
Norcyanine7 Carbamates: CyBams
Cyanine dyes without indolene N-substituents are fluorescent (when protonated), but
derivatives with one a single electronically deactivating substituent are not. Thus when
the Schnermann group (J. Am. Chem. Soc., jacs.1c02112) added carbamates that can
be removed via distal activation the probe lights up. This norcyanine carbamate
(CyBam) probes are actually activated in two ways: first by removal of the para-blocking
group (eg by the enzyme γ-glutamyl transpeptidase (GGT) in tumors) and second by
protonation of the norcyanine component; impressive turn-on ratios (~170x) were
therefore achieved.